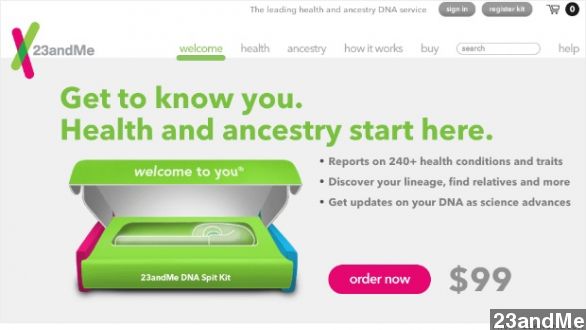
The Food and Drug Administration is putting the brakes on DNA analysis company 23andMe after it advertised its personal genetics kits as medical devices, which is against FDA rules.
23andMe, a genetics firm backed by Google, sells DNA testing kits directly to consumers. The tests reveal customers' ancestry and health information.
"I might have an increased risk of heart disease."
"Arthritis."
"Gallstones."
"Hemochromatosis." ...
"Know more about your health. Go to 23andMe.com and order your DNA kit for only $99 today." (Via 23andMe)
But The Verge notes, 23andMe is making a medical claim about what its product can do — and the FDA requires all medical marketing to be backed up with stringent research.
"If I want to start advertising Cholesterol-Lowering Pills, I have to prove to the FDA that the pills really do lower patients’ cholesterol — usually with a battery of scientific studies that takes years and costs millions ... and 23andMe is way behind in doing the necessary studies to back up their claims." (Via The Verge)
Now the FDA is forcing 23andMe to stop marketing its testing kits. In an unusually snippy letter, the agency notes despite their repeated efforts to work with 23andMe, the company "still had not completed some of the studies and had not even started other studies necessary to support a marketing submission for the [Personal Genetic Service]." It's worth noting, this says nothing of stopping "sales," just of "marketing."
Fast Company says the FDA's move should come as a great relief of insurance companies, who've long worried that personal genetic information could undercut their business.
"It's not hard to imagine people getting their 23andMe results back, finding out their likely to get, say, Alzheimer's, and then rushing out to buy a long-term-care policy. If too many people did that, there would be no business model for long-term-care insurance companies."
But a writer for The Washington Post laments the FDA's decision to shutter 23andMe, arguing consumers have a right to this type of information.
"In a free society, patients have a right to accurate information about their health, even if medical professionals and regulators fear patients will misuse it. That includes information about our genetic code."
Though the decision might not make a difference in the long run. A Slate writer predicts personal genetic tests "are soon going to be as ubiquitous as white bread. ... In hindsight I suspect that the FDA targeting 23andMe is going to seem rather like the RIAA shutting down Napster. The data is coming."
The company responded to the FDA's warning with an official statement promising to address the agency's concerns.